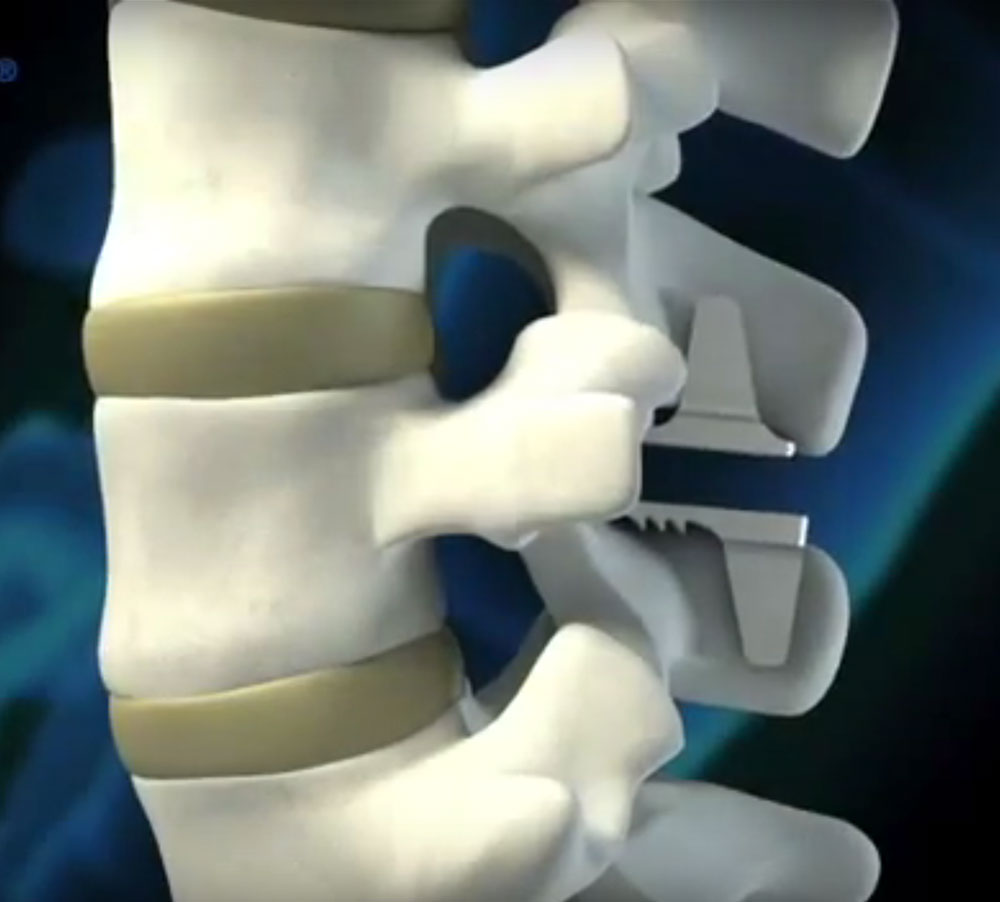
Clinical Need

Potential biomedical materials must clear a high threshold of acceptability in order to gain widespread utilization in implants. Biocompatibility, high corrosion resistance, and superior mechanical properties are essential for candidate materials in order to ensure the health and safety of the patient and the durability and performance of the implant. Ideal materials will not only meet these requirements but allow for good imaging with low artifacts. Currently, industry standard materials for implants include stainless steel, titanium, cobalt chrome, nitinol, tantalum, polyetheretherketone (PEEK). However, many more novel materials could find utilization in this space if biomedical materials testing were streamlined, economical, and able to be performed in-house.